Newsletter
Issue 3 Autumn 2005

|
Newsletter |
 |
|
|
World
Day of Remembrance for Road Traffic Victims 20 November 2005 Worldwide, over 3,000 people are killed and 30,000 injured every day. The price in terms of human loss, grief and care is immeasurable. The impact of such a traumatic event is long-term, often forever. The Day of Remembrance responds to the great need of road crash victims for public recognition, which is so readily given to victims of other types of disaster. It also acknowledges the work of all those involved in the aftermath of a crash – fire, police and ambulance personnel, doctors, nurses and counsellors. |
|
250 PATIENTS
RANDOMISED
|
| CRASH-2 PROGRESS UPDATE | |
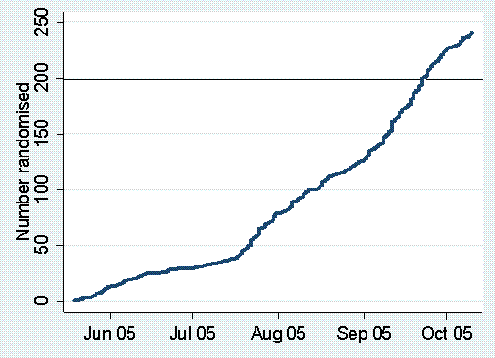 |
Thanks to amazing work by collaborators the CRASH-2 trial has made a spectacular start! We are now randomising patients in 25 hospitals in 10 countries with over 250 patients already randomised. There is every reason to believe that we are on target to deliver a hugely important trial in trauma care. Some facts and figures based on experience so far: |
|
There is massive clinician interest in the CRASH-2 trial: At the time of writing over 300 hospitals from 30 countries have expressed a willingness to take part. Of these more than 80 have already obtained ethics committee approval. Collaborators are getting ethics approval in record time: The interval between the Co-ordinating Centre sending out an ethics pack and ethics committee approval was 20 weeks in CRASH-1 but in CRASH-2 is down to 13 weeks. This shows clearly the advantages of having a network of experienced collaborators. Trial recruitment is well ahead of that in the CRASH-1 trial: For the same interval into the trial we have recruited almost twice as many patients in the CRASH-2 trial. The trial treatment is being given very early: Over two thirds of patients included so far were recruited within 3 hours of the injury. Most of the remaining patients were recruited between 3 and 5 hours following the injury with only a small proportion recruited between 5 and 8 hours following the injury. These data confirm that the treatment can be administered in the emergency setting within a short time of the injury. Early treatment is essential – please keep up the good work. The
characteristics of patients are as we would expect: 86% of trial participants
are males. The mean age of trial participants is 31 years, the median
age is 29 years and the mode is 20 years. About half of the patients had
blunt trauma, the rest having either penetrating trauma alone or a mixture
of blunt and penetrating trauma. In other words… we are on track to deliver a very important trial: If tranexamic acid reduced death and transfusion requirement after trauma this would be a major medical discovery. Only a large scale trial can answer this question. So far we are right on track. The challenge now is to continue to increase the number of participating hospitals and to increase the rate of recruitment at each. EVERY PATIENT COUNTS! |
|
 |
Team
at Mataria Teaching Hospital in Egypt, with Hussein Khamis (centre)
– TOP RECRUITERS! |
|
HUSSEIN
KHAMIS WRITES ABOUT THE EARLY EXPERIENCE OF CRASH2 IN EGYPT: |
|
| Team
at Hospital Luis Vernaza in Ecuador, with Mario Izurieta and Alberto
Daccach (2nd and 3rd from left) – currently second biggest recruiters
with more than 30 patients randomised. |
 |
|
Mario
Izurieta says: Crash2 trial is an learning experience. The
majority of patients that we receive for the trial have penetrating injuries.
Blunt abdominal injury is more challenging because of the results of the
first assessment at admission. Some patients arrive critically ill,
bleeding and showing systolic blood pressure of about 60 mmHg, so
we have to work hard and co-ordinate the efforts to save their lives. The
team in my hospital are developing a great enthusiasm to achieve our
goal – to randomize as many patients as we can. We invite
everyone to join CRASH2, we know we will make a
difference! |
|
|
new collaborators |
|
NEW CENTRES WITH ETHICS APPROVAL CONTINUE TO FLOOD IN. A TOTAL OF 82 HOSPITALS INCLUDE THE FOLLOWING NEWCOMERS: ALBANIA: Arben Banushi, Spitali Civil Durres ARGENTINA: Fernanda Barboza, Hospital Dr Ramón Carrillo COLOMBIA: Arturo Arias, Fundación Clínica Universitaria Santa Catalina; Carlos H Morales Uribe, Hospital Universitario San Vicente de Paul; Luz Elena Flores Rueda and Argemino Gallego Ossa, Hospital General de Medellin; Wilson Gualteros, Hospital Universitario San Jorge; Carlos Rebolledo, Clínica General del Norte CUBA: Marcos Iraloa Ferrer, Hospital Universitario "Dr Gustavo Aldereguia Lima"; Mario Dominguez Perera, Hospital Universitario "Arnaldo Milián Castro" GEORGIA: Gia Tomadze, City Hospital #1 INDIA: P V Ramana, Care Hospital; K V Viswanathan, Medical College, Trivandrum; N K Venkataramana, Manipal Hospital IRAN: Ehsan Sherafat Kazemzadeh, Nemazi Hospital JAPAN: Abe Masayoshi, Fukuoka University Hospital MALAYSIA: Baharudin Bin Abdullah, Hospital University Science Malaysia; April Roslani, University of Malaya Medical Centre MEXICO:Felipe Rendon Elias, Hospital Universitario "Dr Jose E Gonzalez" NIGERIA: Ahmed Olugbenga Sanni, Lagos State University Teaching Hospital; Charles Adeyinka Adisa, Abia State University Teaching Hospital; Lawal Khalid, Ahmadu Bello University Teaching Hospital; Oluwole Olayemi Olaomi, Abuja National Hospital; T O Odebode, University of Ilorin Teaching Hospital POLAND: Adam Mikstacki, Poznan Regional Hospital; Wojciech Gorecki, Krakow University Children's Hospital SAUDI ARABIA: Walid Alyafi and Megahid Eldawlatly, King Khalid National Guard Hospital SINGAPORE: Ivan Nq Hua Bak, National Neuroscience Institute VENEZUELA: Gamal Hamdan Suleiman, Instituto Autonomo Hospital Universitario de los Andes UK: Fiona MacMillan, Furness General Hospital; Kazim Mirza, Colchester General Hospital; Bill Bailey, Chesterfield Royal Hospital; Jeremy Henning, James Cook University Hospital; Fiona Lecky, Hope Hospital; Suzanne Brady, Worthing Hospital |
|
new randomisers – congratulations to all! |
|
The teams in all these hospitals have managed to get over the first hurdle of identifying their first eligible patient. Be brave – just do it! Randomisation will get easier after the first one.
|
|
|
|
|