Newsletter
Issue 15 - May 2009

|
Newsletter |
 |
|
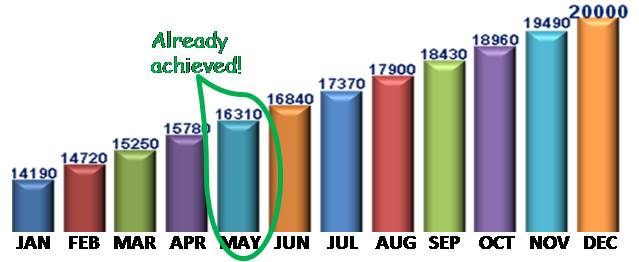
ON TARGET!
|
|
|
THIS
IS HOW WE DO IT:
|
|
|
THE SUCCESS OF THE CRASH-2 RANDOMISATION IN NATIONAL HOSPITAL ABUJA, NIGERIA, IS DUE TO COLLECTIVE EFFORTS OF EVERYBODY WHO HAS RECRUITED PATIENTS INTO THE STUDY The nature of National Hospital Abuja, Nigeria, CRASH-2 team is very fluid; everybody who has worked and those still working in the Accident & Emergency unit are part of the team, particularly casualty doctors, surgical residents on rotation in the trauma team or on call duty in the unit. There is regular monitoring and reminders by the principal investigator and the head nurse. CRASH-2 is a regular announcement item in every meeting of the unit and regular incentives are given at monthly intervals to the highest recruiter of the month using some of the items sent to us from the TCC. CRASH-2 enjoys the support of the hospital management, all the consultants in the Department of Surgery and above all the Principal Investigator, who takes CRASH-2 as a personal project giving it all the attention it deserves, carrying everybody along to ensure that all eligible patients are recruited into the trial. |
|
|
TBILISI STATE UNIVERSITY CLINICAL HOSPITAL 'I JAVAKHISHVILI' GEORGIA, IS SITUATED IN THE CENTRAL AREA OF TBILISI, NEAR THE RIVER MTKVARI AND TWO MAIN HIGHWAYS ON BOTH SIDES OF THE RIVER. There are different departments in our clinic for managing emergency patients: Surgery, Neurosurgery, Orthopaedic, Maxillofacial surgery and Intensive Care unit. Many patients attend our clinic with different kinds of injury, complicated with bleeding or with high risk of bleeding. Sometimes we have a big problem finding the necessary blood group. This is why this trial awakened a great interest for us. Using the effective solution against blood loss or as prophylaxis seems very attractive. For that reason we still continue randomising patients with great enthusiasm. Despite the final results of the trial, we are enjoying the communication with the TCC team – it has been a great experience for us. We hope, that our contribution will help to find the answer to the trial question. |
 Buba Shalamberidze |
| 1,000 patient team | ||
|
Here are the wonderful people at Hospital Universitario San Vicente de Paul in Medellin, Colombia, who have made their hospital the first to recruit 1,000 patients into the trial. Huge thanks and congratulations to all! |
||
|
|
 |
 |

| MENTIONS – Congratulations and thanks to each one of the teams listed here for their great achievements: | |
|
|
Jorge Augusto Herrera Chaparro Hospital Universitario San Jose de Popayan, Colombia Tamar Gogichaishvili, Tbilisi First Hospital, University Clinic, Neurosurgery Center, Georgia Wole Olaomi, National Hospital Abuja, Nigeria |
 |
|
 |
|
 |
|
 |
|
 |
|
|
TOP RECRUITERS OVERALL
|
|
|
TOP AVERAGE MONTHLY RECRUITMENT
|
|
CRASH-2 AROUND THE WORLD |
|
|
Team at Hospital Rovirosa, Mexico, with PI Raul Bautista Cruz |
|
|
PI Yusuf Adamu Al-Hassan and Alhaji Ishak from Nyinahin Government Hospital Ghana
|
Tbilisi State University Clinical Hospital 'I Javakhishvili' Georgia, PI Buba Shalamberidze |
|
Team at Aditya Neuroscience Centre, India, with PI Anil P Lal, within the top 10 recruiters by monthly average. |
Team at Hospital Pablo Tobon Uribe, Colombia, with PI Alfredo Constain. |
|
Our Nigeria national co-ordinator Edward Komolafe was a guest speaker at the UK national meeting held in March in Leicester. |
|
|
Luis Barrera from Hospital Universitario San Vicente de Paul, Colombia, spent some time in London and delighted the team at TCC with his visit. |
|
| TEAM NEWS | |
|
Collette Barrow joined the team in November 2008 as an assistant trials administrator. She says: "I work with the admin team making sure that drugs and expenses payments get to collaborating hospitals without delay. My background is administrative and I have worked within a number of different industries with over 25 years of experience. It is great to be working with the Trials Coordinating Centre team and being involved in this exciting and interesting investigative work." |
 |
| Many of you will already be familiar with Chris Rubery who started in February as a data assistant. He says, "I've worked in data management for clinical trials since leaving university two years ago, but CRASH-2 is my first trial with an international focus, so I am enjoying the opportunity to expand my horizons by talking to people in all the many countries around the world the trial collaborates with." |  |
|
WHY IS IT IMPORTANT THAT YOUR DATA ARRIVES ON TIME? »» Please send entry form as soon as possible after randomisation »» We need to know about randomised patients so that they are covered by our trial insurance »» It is vital that the outcomes of patients who have received the trial treatment are known as soon as possible; please send outcome forms as soon as they are due »» The data team will contact you individually with lists of outstanding forms Thank you to all who have responded to our queries about type of injury and date of discharge errors. If you haven't yet responded there is still time… |
| cochrane corner | |
 |
The Cochrane Injuries Group editorial base is located at the London School of Hygiene & Tropical Medicine in the UK. Our work involves preparing, maintaining and promoting the accessibility of systematic reviews in the prevention, treatment and rehabilitation of traumatic injury, including the emergency resuscitation of seriously injured and burned patients. The Cochrane Injuries Group (CIG) has an international membership and editorial team. Our members include researchers, health care professionals, people using health services (consumers) and other interested parties who are involved in the working of the group in a variety of ways – from hand searching journals for relevant trials to translating trial titles and abstracts into English. Cochrane Library (www3.interscience.wiley.com); Spanish language translations are available at www.bibliotecacochrane.net/Clibplus/ClibPlus.asp. For further information contact Emma.Sydenham@Lshtm.ac.uk or log into www.injuries.cochrane.org |