Newsletter
Issue 11 Autumn 2007

|
Newsletter |
 |
| 7,000
RANDOMISED PATIENTS | |
|
Dear Collaborators, As the year 2007 ends, we would like to let you know of some of the fantastic achievements you have had in your trial over the last year, and our plans for 2008. It seems a long time ago when in our February newsletter we were able to report that HTA had agreed to fund the trial. This gave us the security to push on with our task of recruiting patients. By the end of this year over 7,000 patients will have been recruited. This means we were able to recruit over 4,000 patients in 2007 alone. You have already made CRASH-2 the largest trial in trauma patients with bleeding ever conducted. Our collaborative group has grown even bigger and now includes over 150 active hospitals in 33 countries. | 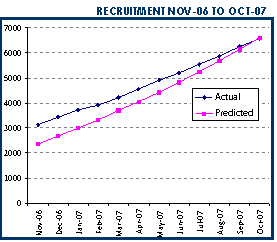 |
National collaborator meetings have been held in various countries this year and we would like to thank all the investigators who give freely of their time and experience to make these meetings successful. Together we are making a huge change in the paradigm of trauma care research and we are showing that it is possible to carry out large, high quality, research projects on relevant clinical questions. So congratulations to all! Our target for next year is to double the recruitment, so we will need 8,000 patients recruited (no easy target)! We know that this is very achievable as many of you have been inviting others to join the collaboration. We still need the help of all our collaborators to spread the word to others. Congratulations again for a successful 2007 and we look forward to the next year! From your coordinating team | |
|
 National Meeting in Peru in November 2007 |
 National meeting in Cuba in May 2007 |
|
GEORGIA NO CONSENT APPROVAL A successful national meeting was also held in Georgia in May. The Georgian drug agency have just given their approval to recruit patients into the trial without prior written consent. We hope that this ruling will significantly increase the patient recruitment in the three active centres in Georgia, and hopefully encourage new hospitals to join. To date 253 patients have been randomised in Georgia. |
STAR RECRUITERS |
500 50 RANDOMISED PATIENTS
|
|
new ethics approvals:
|

| started randomisation:
|
Background
image: Bhattacharya Hospital, India |
cochrane
corner |  |
| The
systematic review about the use of an anti-fibrinolytic for minimising the need
for perioperative allogeneic blood transfusion (Henry et al.) has been recently
updated. This update included 211 randomised controlled trials that recruited
20,781 patients. The authors’ main conclusions were that anti-fibrinolytic
drugs provide worthwhile reductions in blood loss and the need for allogeneic
red cell transfusion, that efficacy does not appear to be offset by serious adverse
events, and that tranexamic acid is probably as effective as aprotinin. The full text of the review can be accessed through The Cochrane Library (www.thecochranelibrary.com). For more information about the Cochrane Injuries Group and to access abstracts of other systematic reviews, please visit www.cochrane-injuries.Lshtm.ac.uk. Reference: | |
I started working as an Assistant Trials Manager in September. My background is in laboratory research and I have a PhD in molecular biology. One of my roles in the CRASH-2 trial is to assist our collaborators in India and Sri Lanka and I hope to help make the trial a success in these countries. I’m enjoying working on a clinical study and am happy to be part of such an important international collaboration. |  Taemi Kawahara |
 Lisa Cook | I have just joined the CRASH-2 team as an Assistant Trials Manager. I will be helping collaborators in Asia, Australasia and parts of Eastern Europe with all aspects of the trial. I have previously worked in HIV clinical trials, but working in trauma is a new challenge for me. I am very much looking forward to working on such a large and important international trial, and to getting to know collaborators around the world! |